New medications coming soon: Here’s what’s been FDA approved in 2025 so far
New medications coming soon: Here’s what’s been FDA approved in 2025 so far
Each year, the a wide range of medications that help shape the future of medicine in the U.S. These include novel drugs that offer new treatment options, as well as first generics and biosimilars that aim to help lower costs and improve access.
, a platform for medication savings, tracks these approval trends, offering insight into what’s new, what’s going generic, and how the treatment landscape is changing. These patterns may also be influenced by broader factors, such as regulatory reforms, administrative priorities, and the pace of scientific discovery.
Key takeaways:
- The FDA approves a range of medications each year, including novel drugs, first generics, biosimilars, and more. Each category plays a unique role in healthcare innovation, cost savings, and access.
- During the first half of 2025, novel drug and biosimilar approvals have paced slightly behind the numbers from this time in 2024. So far, the first generic approval numbers are ahead compared to 2024, which itself lagged behind prior record-setting years.
- In late 2024, the FDA finalized a new rule for switching prescription drugs to over the counter (OTC), which went into effect May 27, 2025. This new rule will expand the types of medications that can make the switch, such as statins and erectile dysfunction pills.
Novel drug approvals in 2025
Novel drugs are brand-new medications containing that have never been FDA-approved before. These therapies often address an unmet medical need or offer meaningful advances over existing treatments. Examples include breakthrough cancer therapies, new antibiotic classes, or first-in-class diabetes medications.
Novel drugs typically go through the (NDA) or (BLA) process, which involves to show they’re safe and effective. Some also receive designations such as priority review, , or , which can speed up the FDA review and approval process.
Over the last 10 years, the number of has varied from year to year:
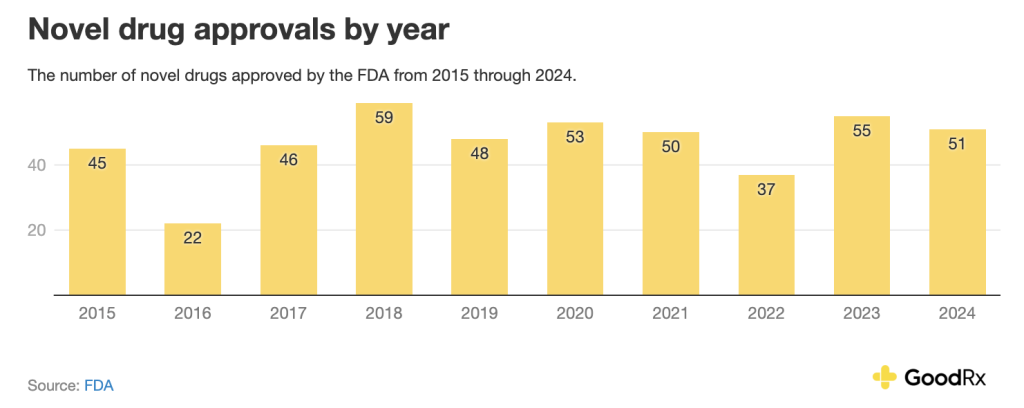
As of , CDER has approved 16 novel drugs this calendar year. This is slightly behind pace compared to this time last year. Recently, the FDA announced that it plans to use a few different strategies to streamline the drug approval process in certain cases. These include the and a new (CNPV). The CNPV program intends to shorten the agency’s review time from 10 to 12 months to one to two months.
Here is a quarterly comparison of novel drug approvals between 2025 and 2024:
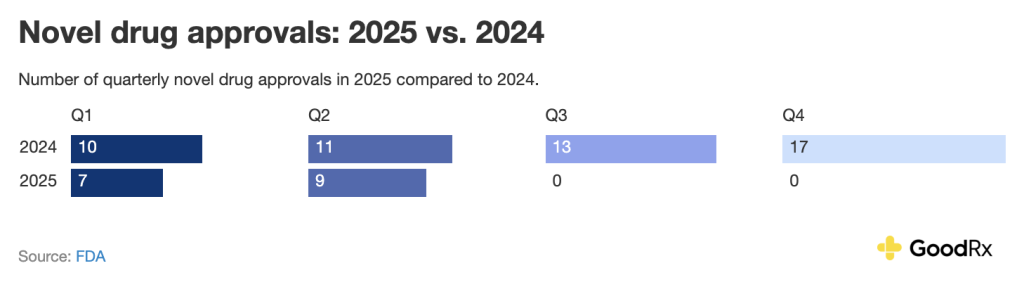
Several high-impact medications, especially in oncology, neurology, and infectious disease, are in the pipeline for potential . Here are the 2025 novel drug approvals as of late June.
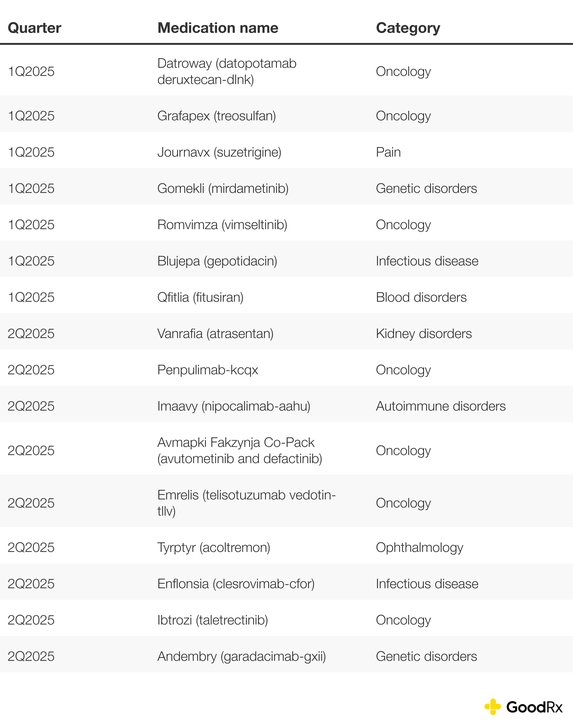
Note: The numbers in this section reflect novel drug approvals by the FDA’s Center for Drug Evaluation and Research (CDER). They do not include approvals for vaccines, cellular or gene therapy products, or other products approved by the FDA’s Center for Biologics Evaluation and Research (CBER).
First generic approvals in 2025
Once a brand-name medication is no longer protected by patents or exclusivity, manufacturers are able to market of it. The “first generic” is the very first FDA-approved copy of a brand-name medication. A first generic for six months of exclusivity, meaning that it’ll be the only generic on the market during this time. Other generics can typically enter the scene after this.
First generics (and any subsequent generics) are approved through the (ANDA) process. They don’t typically need to repeat clinical trials for approval. Instead, manufacturers are required to show that the generic is therapeutically equivalent to the brand-name drug. In other words, you can expect the same results.
Over the last 10 years, first generic approvals have also varied:
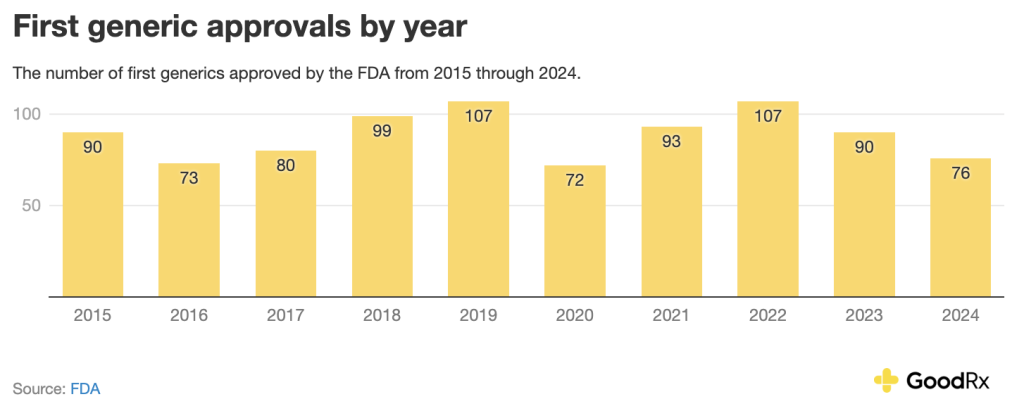
As of late June 2025, at least 44 first generics . Keep in mind that first generics may not be available right after approval because of patent protections. They often launch years later.
Examples of 2025 first generic approvals include:

Note: First generic approval numbers from 2015 through September 2024 are from the FDA’s . The fourth quarter 2024 numbers are from the FDA’s report. First generic approvals for 2025 are from the Orange Book Cumulative Supplement. Total 2025 numbers may change as the FDA approves more first generics and updates their reporting for year-to-date approvals.
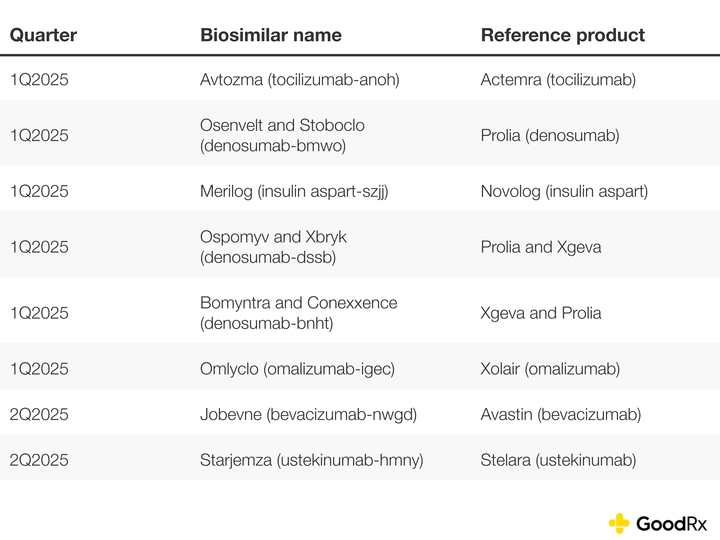
Biosimilar approvals in 2025
represent a broad group of complex medications that are made using living systems, . Biosimilars are of another biologic medication (called the reference product). with brand-name drugs, biosimilars can potentially help you save money on your biologic prescription.
Biosimilars are approved through the , which involves showing that there are no clinically meaningful differences between the biosimilar and the reference product. Some biosimilars may go through additional studies to show that they’re another biologic. This designation can make it easier to switch to a biosimilar at the pharmacy counter (depending on state law).
have fluctuated over the recent years, with 2024 as a record-setting year:
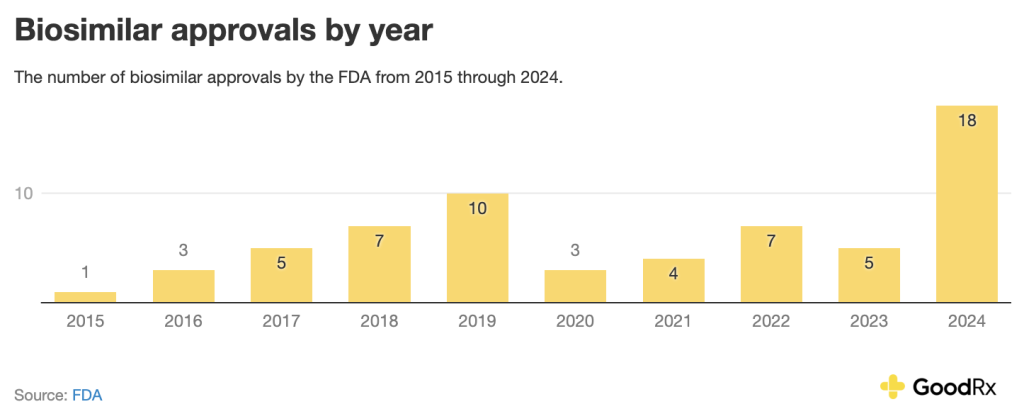
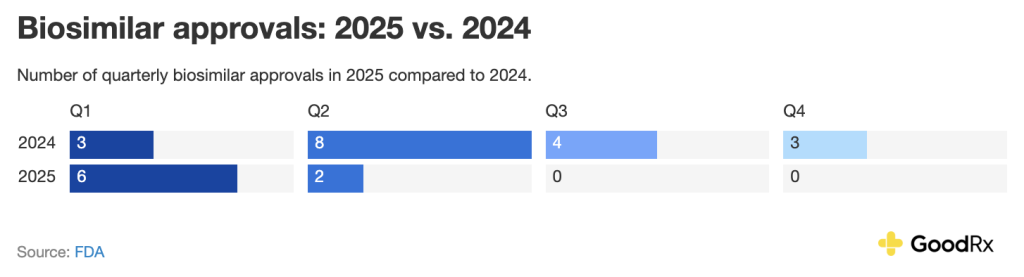
So far in 2025, there have been eight biosimilar approvals. Here’s how this number compares to 2024 on a quarterly basis:
Here are the 2025 biosimilar approvals so far:
Rx-to-OTC switches
An takes place when a prescription medication is approved for over-the-counter (OTC) use, meaning that you can buy it without a prescription. Through this process, the FDA determines that a medication can be safely and effectively used to self-treat symptoms with clear, easy-to-understand labeling.
Rx-to-OTC switches are typically approved through an NDA process. They can be full switches, where the OTC product is marketed for all the same uses as the prescription product. Partial switches, on the other hand, involve only some of the same uses. With either type, the manufacturer needs to show that the product can be safely used without medical supervision.
Rx-to-OTC switches are as other types of approvals. But when these switches are approved, they can have significant public health impacts. (naloxone), an opioid overdose treatment, and (norgestrel), the first OTC birth control pill, are two recent examples of such approvals.
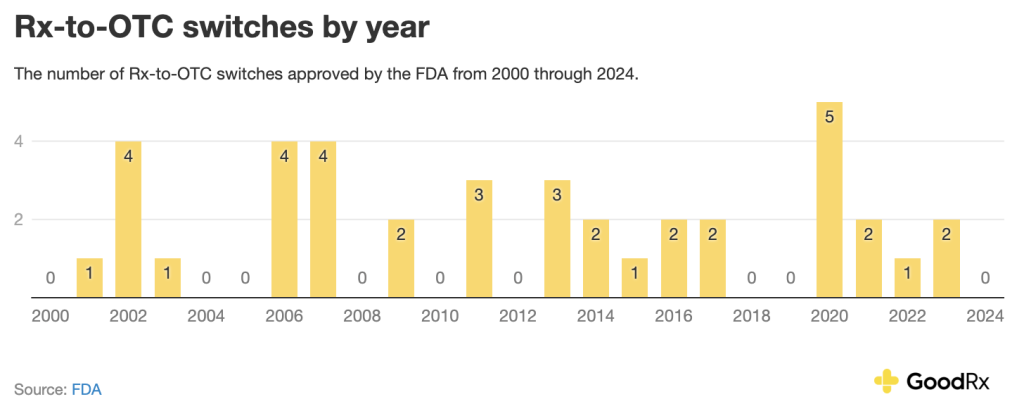
At the end of 2024, the FDA that would of medications that could be eligible for an Rx-to-OTC switch, such as and . After a , this rule went into effect on May 27, 2025. The FDA has not approved any Rx-to-OTC switches so far in 2025.
The bottom line
Every year, the FDA approves novel drugs, biosimilars, and first generics. Compared to 2024, novel drug and biosimilar approval numbers are slightly behind in the first half of 2025. First generic approval numbers are pacing ahead.
was produced by and reviewed and distributed by Â鶹Դ´.